Add to Cart
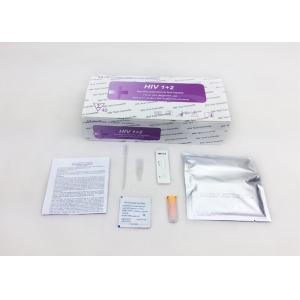
HIV Self Test kit , Certified, Whole Blood/Serum/Plasma Specimen, Home test use for HIV antibody
Accessories:
| Test Cassettes | Antiseptic wipe | Sterile lancet |
| Pippette | Sterile gauze pad | Package Insert |
| Buffer |
INTENDED USE:
The HIV self Test kit (Whole Blood/Serum/Plasma) is a rapid chromatographic immunoassay for the qualitative detection of antibodies to Human Immunodeficiency Virus(HIV) type 1 and type 2 in whole blood, serum or plasma to aid in the diagnosis of HIV infection. It is easily for personally use at home.
SUMMARY AND EXPLANATION OF THE TEST
HIV is the etiologic agent of Acquired Immune Deficiency Syndrome (AIDS). The virion is surrounded by a lipid envelope that is derived from host cell membrane. Several viral glycoproteins are on the envelope. Each virus contains two copies of positive-sense genomic RNAs. HIV 1 has been isolated from patients with AIDS and AIDS-related complex, and from healthy people with high potential risk for developing AIDS.1 HIV 2 has been isolated from West African AIDS patients and from seropositive asymptomatic individuals.2 Both HIV 1 and HIV 2 elicit immune response.3 Detection of HIV antibodies in serum, plasma is the most efficient and common way to determine whether an individual has been exposed to HIV and to screen blood and blood products for HIV.4 Despite the differences in their biological characteristics, serological activities and genome sequences, HIV 1 and HIV 2 show strong antigenic cross-reactivity.5,6 Most HIV 2 positive sera can be identified by using HIV 1 based serological tests. The HIV 1.2 Rapid Test Cassette(Whole Blood/Serum/Plasma) is a rapid test to qualitatively detect the presence of antibody to HIV 1 and/or HIV 2 in whole blood, serum or plasma specimen. The test utilizes latex conjugate and multiple recombinant HIV proteins to selectively detect antibodies to the HIV 1.2 in whole blood, serum or plasma.
Test Principle
The HIV 1.2 Rapid Test Cassette (Whole Blood/Serum/Plasma) is a qualitative, membrane based immunoassay for the detection of antibodies to HIV 1.2 in whole blood, serum or plasma. The membrane is pre-coated with recombinant HIV antigens. During testing, the whole blood, serum or plasma specimen reacts with HIV antigen coated particles in the test Dipstick. The mixture then migrates upward on the membrane chromatographically by capillary action and reacts with recombinant HIV antigen on the membrane in the test line region. If the specimen contains antibodies to HIV 1 and/or HIV 2, a colored line will appear in the test line region, indicating a positive result. If the specimen does not contain HIV 1 and/or HIV 2 antibodies, a colored line will not appear in the test line region, indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that proper volume of specimen has been added and membrane wicking has occurred.
TEST PROCEDURE
Allow the test, specimen, buffer and/or controls to reach room temperature (15‐30℃) prior to testing.
1. Bring the pouch to room temperature before opening it. Remove the test cassette from the sealed pouch and use it as soon as possible.
2. Place the cassette on a clean and level surface.
For Venipuncture Whole Blood specimen: Hold the dropper vertically and transfer 2 drops of whole blood (approximately 50 mL) to the specimen area, then add 2 drops of buffer (approximately 80mL), and start the timer. See illustration below.
For Fingerstick Whole Blood specimen:
INTERPRETATION OF RESULTS
Negative: The presence of only one line in the control region indicates a negative result (Figure 1).
HIV-1 Positive: The control line and HIV-1 line (T1) are visible in the result window. The test is positive for HIV-1.
HIV-2 Positive: The control line and HIV-2 line (T2) are visible in the result window. The test is positive for HIV-2.
HIV-1 and HIV-2 Positive: The control line, HIV-1 (T1) and HIV-2 (T2) lines are visible in the result window. The test is positive for HIV-1and HIV-2.
Regarding the positive results for both HIV-1 and HIV-2 in one patient, it is possible for reasons as follows:
1. There is the homology in the amino acid sequence of HIV type-1 and type-2. So, it is possible that the test
results show the positive results for HIV-1 and HIV-2 in one patient, simultaneously.
2. Provisionally, you can conclude virus type according to the line density. If the line density of type-1 is darker
than that of type-2 in the result window, you can read as HIV-1 positive. If the line density of type-2 is darker
than that of type-1 in the result window, you can read as HIV-2 positive. If you want to determine virus type or co-infection exactly, you should perform the confirmatory assay (e.g Western blot etc.).
Invalid: If the pink color line in C region is not visible, the result is considered invalid (Figure 3) regardless of the presence or absence of the test line(s).
Interfering Substance
Analytes were spiked into negative plasma and serum pools (ELISA confirmed) and low positive plasma and serum specimens (ELISA confirmed) at the concentrations listed. The specimens were tested in triplicate with visual interpretations occurring at 10 and 20 minutes after specimen application. Results are presented in table below.
Table: Interfering Substance
| HIV14050004‐T | HIV14050005‐T | HIV14050006‐T | ||||||||||||||
| Concentratio | ||||||||||||||||
| Analytes | Plasma | Serum | Plasma | Serum | Plasma | Serum | ||||||||||
| n | ||||||||||||||||
| Neg | P‐ | Neg | S‐ | Neg | P‐ | Neg | S‐ | Neg | P‐ | Neg | S‐ | |||||
| 0 | 0 | 0 | 0 | 0 | ||||||||||||
| . | 03 | . | . | . | . | . | ||||||||||
| 5 | 3 | 5 | 3 | 5 | ||||||||||||
| Ascorbic acid | 20mg/ml | ‐* | +* | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | |||
| * | ||||||||||||||||
| Hemoglobin | 1,100mg/dl | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | |||
| Gentistic acid | 20mg/dl | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | |||
| Oxalic acid | 600mg/dl | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | |||
| Bilirubin | 1000mg/dl | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | |||
| Acetaminophe | 20mg/dl | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | |||
| n | ||||||||||||||||
| Acetylsalicylic | 20mg/dl | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | |||
| Acid | ||||||||||||||||
| Creatine | 200mg/dl | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | |||
| Albumin | 2000mg/dl | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | |||
| Caffeine | 20mg/dl | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | ‐ | + | |||
| Note: “*” mean negative result | “**” mean positive result | |||||||||||||||
Conclusion:
As per the results tabulated above, no substances showed any interference with the expected test results within the specified result interpretation time of 10 to 20 minutes.
| ORIENT NEW LIFE MEDICAL CO., LTD. | |
| Contact: | Jerry Meng |
| Email: | Jerry @ newlifebiotest .com |
| Tel. | +86 18657312116 |
| SKYPE | enetjerry |